Date July 7, 2023 | Brooke Loeffler
How Do Deicers Affect Biochemical Oxygen Demand?
What is Dissolved Oxygen?
First, we need to start off with learning about dissolved oxygen. Dissolved oxygen (DO) is the measure of how much O2 is dissolved within a body of water. This oxygen allows aquatic organisms like fish and zooplankton to breathe. Oxygen can enter a body of water in a few different ways:
- Photosynthesizing aquatic plants
- Diffusion at the surface
- Aeration from flowing and churning water

There are a few other variables that affect how much DO is contained within a body of water. Faster currents, cooler water temperatures, and lower levels of organic matter all increase DO levels.
On the opposite side of the spectrum, stagnant, warm water, with high levels of rotting organic material contain low levels of DO. Under these conditions, bodies of water can become eutrophic (nutrient rich and oxygen depleted), form toxic algae blooms, grow noxious bacterial slimes, and kill off large quantities of aquatic life.
What is Biochemical Oxygen Demand (BOD)?
So how do environmental scientists know when a body of water is changing from aerobic (oxygen rich) to eutrophic? They measure the biochemical oxygen demand.

Biochemical oxygen demand (BOD) is the amount of oxygen that is demanded by microorganisms in order to break down excess levels of nutrients and organic materials. Healthy, aerobic bodies of water contain a proper balance of dissolved oxygen, micro-organisms, and organic materials.
Water health can deteriorate if too many nutrients flow into the water. When organic material levels are too high, microorganisms get to work breaking down those nutrients. During this breakdown, microorganisms also consume dissolved oxygen in the water.
How is BOD Measured?
BOD is measured in milligrams of oxygen consumed per liter of water. Scientists take water samples and incubate them for 5 days at 68° F/20° C.

What Causes High BOD levels?
According to NOAA:
“Sixty-five percent of the estuaries and coastal waters in the contiguous U.S. that have been studied by researchers are moderately to severely degraded by excessive nutrient inputs.”
These dead zones have been appearing in the news more frequently in recent years, and they can form under both natural and human-induced stresses.
Natural stresses can include:
- High summer temperatures
- Water flow blockages due to natural disasters, etc.
Human caused stresses that create oxygen depleted zones are most commonly caused by:
- Fertilizer runoff (mainly high levels of nitrogen and phosphorus)
- Overuse of certain deicing compounds that collect in gulfs, ponds, lakes, and estuaries.
How Do Deicers Affect BOD?
Deicers can contain a few different compounds to lower the freezing temperature of water. The most common deicing compounds are:
- Chloride Salts (sodium chloride, calcium chloride, magnesium chloride, potassium chloride)
- Acetates (like calcium magnesium acetate)
- Glycol fluids (ethylene or propylene glycol-water based solutions)
- Agricultural byproducts (including fermented concentrated beet pulp and corn derivatives like Caliber and Ice Ban)
Let’s look at each of these categories and their effect on biochemical oxygen levels.
Chloride Salts
Chlorides are the most common type of deicing compound used today. They are used in both solid granular and liquid form. According to a government deicing impact study conducted by the Idaho Transportation Department:
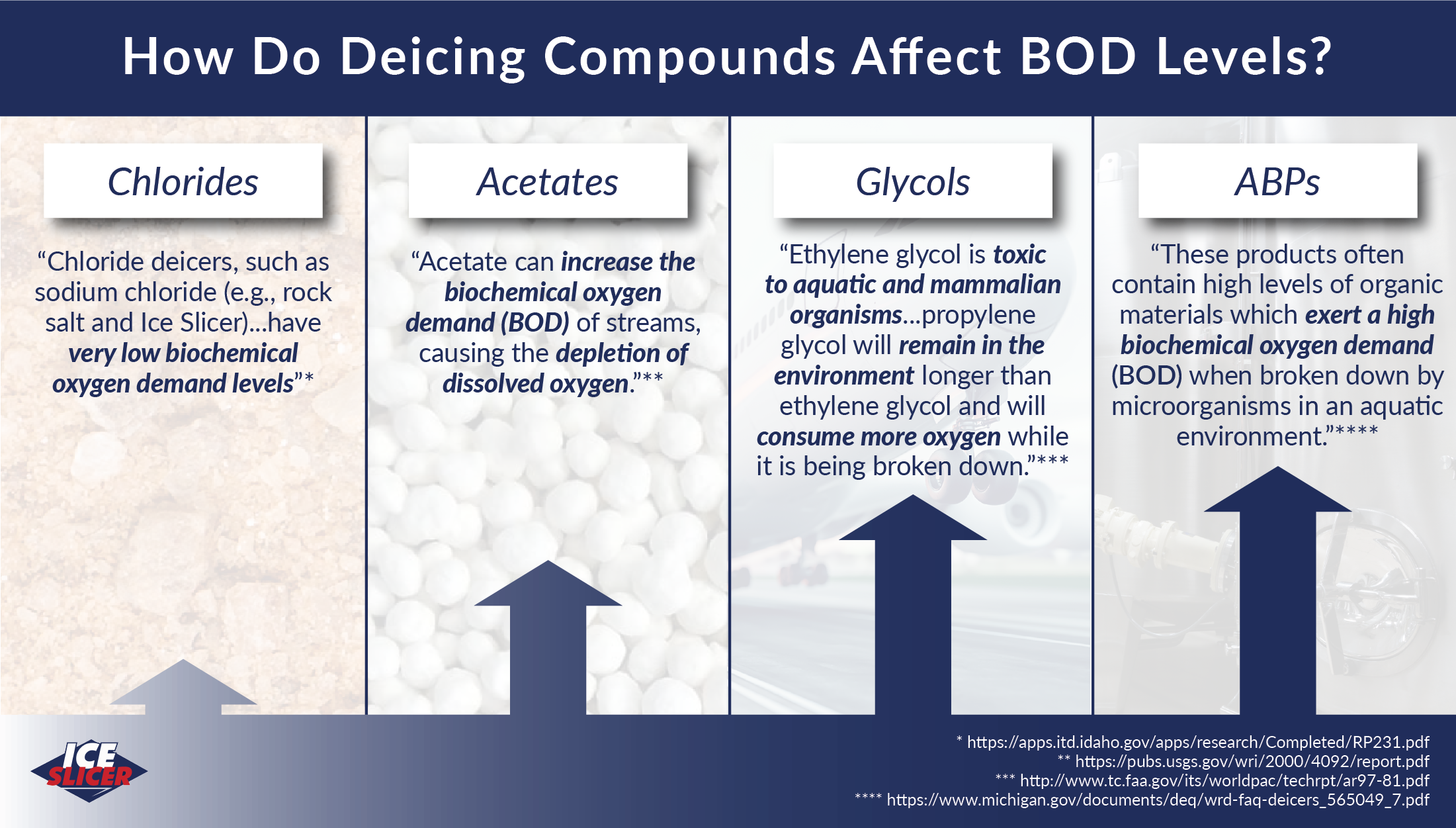
“chloride deicers, such as sodium chloride (e.g., rock salt and Ice Slicer)...have very low biochemical oxygen demand levels.”
Some products add acetates and agricultural byproducts to try and enhance chloride deicing performance or decrease corrosion. Let’s see how these additives change deicers’ impact on BOD levels.
Acetates (Calcium Magnesium Acetate)
Acetates, such as calcium magnesium acetate, have organic molecules that can be used by microorganisms as nutrients. The United States Geological Survey (USGS) states:
“the adverse environmental effects of CMA used as an anti-icing material are mainly related to the acetate ion, C2H3O2. Acetate can increase the biochemical oxygen demand (BOD) of streams, causing the depletion of dissolved oxygen.”
Glycols (Ethylene and Propylene Glycol Solutions)
Glycol solutions are most commonly used to deice aircraft. Since glycol is a sugary alcohol, you can imagine the harmful microbial feast it triggers when it reaches waterways. The Federal Aviation Agency (FAA) has admitted that:
“ethylene glycol is toxic to aquatic and mammalian organisms” and that “propylene glycol will remain in the environment longer than ethylene glycol and will consume more oxygen while it is being broken down. Therefore, it can still be harmful to the environment.”
Because of these risks, the FAA has been conducting lab and field tests in bioremediation to reduce these effects “using various combinations of inocula (bacteria), nutrient mixes, enzyme mixes, and ultrasonic stimulation.”
Agricultural Byproducts (ABPs)
Agricultural byproducts from beets and corn can be fermented and used as deicing compounds. These compounds are sometimes added to other deicers as corrosion inhibitors to reduce levels of corrosion on infrastructure. These byproducts have been shown to help vegetation along the roadside but are also extremely detrimental to waterways and aquatic life. The Michigan Department of Environmental Quality has been studying the effects of agricultural byproduct deicers on their many lakes and slow flow streams. They found that:
“de-icing products derived from ABPs have the potential to adversely affect water quality if allowed to enter surface waters. These products often contain high levels of organic materials which exert a high biochemical oxygen demand (BOD) when broken down by microorganisms in an aquatic environment. This results in reduced in-stream levels of dissolved oxygen (DO), which is necessary for the survival of aquatic life. Fish kills, impaired biological communities, and noxious growths of bacterial slimes can result from elevated BOD and reduced levels of DO in streams and lakes.
Some ABP deicers have the potential to greatly impact DO concentrations in surface waters, as they may contain many times the amount of BOD found in strong wastes like raw sewage.”
The study mentioned earlier, conducted by the State of Idaho, found that:
“organic matter, such as CSB [concentrated sugar beets] used as a corrosion inhibitor, can increase biochemical oxygen demand in receiving waters, depriving aquatic life of oxygen.”
They also noted that:
“agricultural byproducts patented for use as roadway deicing and anti-icing agents, including the liquid residue of fermented corn byproducts...has been shown to cause ‘eutrophication of water, which will result in the proliferation of noxious aquatic plants, especially blue green algae,’ reducing water oxygen levels.”
The Ice Slicer® Advantage
So how do we balance the need to restore road safety during the winter with protecting our precious waterways? Well, we are proud to say Ice Slicer® granular deicer is the answer. Mined from a Jurassic mineral deposit in Utah, Ice Slicer® is naturally high-performing without the need for harmful additives. Our products do not contain any acetates, glycols, or agricultural byproducts that lead to detrimental biochemical oxygen demand in our waterways. Our road treatment is an all natural blend of complex chlorides (all 4 chloride salts) and over 60 trace minerals. Ice Slicer® is a naturally powerful and environmentally friendly alternative to the more destructive deicers and salts on the market. Learn more about winter road treatments and waterway health, and see how Ice Slicer® stacks up against other methods.
© 2024 Redmond Minerals Inc.