Date December 20, 2022 | Brooke Loeffler
The Chemistry of Deicing: How Does Ice Melt Work?
The primary goal of any deicing product is to reduce the freezing point of water and form a brine solution. A solution consists of a solvent (liquid) and a solute (a solid that dissolves into the liquid). Deicers contain solute ingredients that lower the freezing point of water as they dissolve into melting snow/ice. There are many freezing point depressants, and those found most frequently in deicers belong to a family of chloride salts.
Meet the Chlorides
Sodium Chloride (or white salt) is the most common salt found in deicing products. Other salts such as magnesium chloride, potassium chloride, and calcium chloride can also be present. When each of these compounds come in contact with any form of water (including ice), they lower its freezing point.

Each chloride depresses water’s freezing temperature to a different point:
- Sodium Chloride lowers to about 21° F / -6° C
- Potassium Chloride lowers to about 12° F / -11° C
- Magnesium Chloride lowers to about -5° F / -15° C
- Calcium Chloride lowers to about -25 ° F / -32° C
Click here to learn more about the difference between the working and eutectic temperatures of these salt solutions.
A Caution About Additives
Some deicing products include dyes and liquid additives to increase performance and make it easier to see where the product has been applied.
Added Liquid Chlorides
These products will typically take sodium chloride (white salt) and add liquid magnesium, potassium, or calcium chloride to boost brine making power down to lower temperatures.
When liquid additives are combined with solid salt crystals, they just coat those crystals instead of permeating them. These liquids leach out quickly and do not deliver consistent, long lasting deicing power.

Fast leaching liquid chlorides also increase infrastructure and vehicle corrosion for both concrete and metal surfaces. Products with added liquid chlorides are also more environmentally damaging because they leach larger amounts of chlorides into the soil. These leaching chlorides bind with soil particles and prevent plants from absorbing nutrients and water. They also activate heavy metals in the soil that can harm groundwater health.
Added Dyes
In order to see where a deicer has already been applied, some products add dyes to their mix. These added dyes stain and damage concrete and can also harm the environment. According to the EPA substances found in dyes can bio-accumulate and “dyes tend to absorb ultraviolet radiation and have the potential to form decay products more toxic than the original parent dye compound.”

The Chemistry of Ice Slicer®
Ice Slicer® granules naturally contain a mix of complex chlorides and minerals for deicing power that outperforms white salt every time. Mined from an ancient sea deposit in Utah, our naturally reddish granules don’t need any additional dyes to help with application.
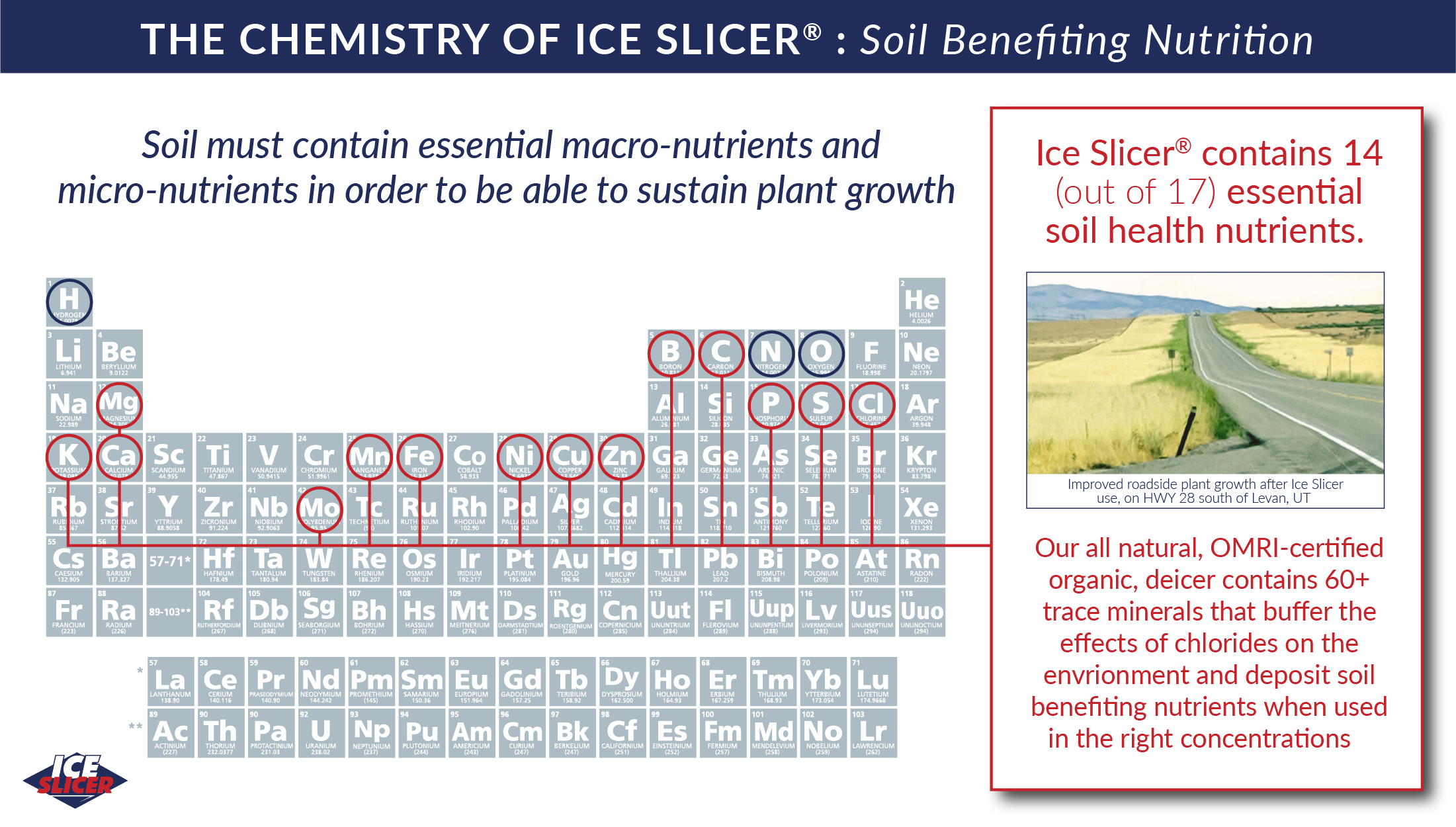
In addition to the high-performing chlorides necessary to form brine, Ice Slicer® contains over 60 trace minerals that buffer the effects of chlorides on the roadside environment. In the correct concentrations, these trace minerals not only act as an environmental buffer, they also encourage healthier plant growth. The chemistry speaks for itself...Ice Slicer® delivers fast and powerful deicing performance while reducing unnecessary chemical damage to the environment.
© 2024 Redmond Minerals Inc.





